Targeting Cancer Stem-Cell Proteins Might Be Key to Defeating Therapy-Resistant Cancers
Dr. Tamara Abou Antoun, an assistant professor in the Pharmaceutical Sciences Department at the School of Pharmacy, has been focusing her attention on highly malignant cancer stem cells that survive current therapies and regenerate tumors. She is currently working in collaboration with Dr. Jad Abdallah, Dr. George Khazen, and Dr. Zeina Nasr (University of Balamand). In this article Dr. Abou Antoun explains the key findings, observations and trajectory of their research.
• • •
Despite advances in treatment options, tumor recurrence with metastatic malignancies remains a great challenge, with fatal outcomes in cancer patients. Cancers that recur are usually characterized by poor prognosis and resistance to therapy.
Our work is mainly focused on pediatric neuro-oncology tumor models. We aim to identify the tumorigenic proteins and pathways that govern malignant, treatment-evasive behaviors in childhood brain cancers through molecular and proteomic approaches.
The currently available treatment options target the bulky tumor mass, leaving behind highly malignant, tumor-initiating stem-like cells often referred to as “tumorspheres.” Moreover, radiotherapy, used as a standard approach for most cancers, has been shown to induce upregulation (increase) of highly tumorigenic, cancer stem-cell driving, and therapy-resistance proteins such as L1-CAM (Rached et al. Int. J Oncol. 2016) that may be playing a pivotal role in the malignant nature of these aggressive cancers.
Recurrent cancer has a therapy-resistant phenotype that renders it unresponsive to current treatment options. It is believed that tumorspheres possess the ability, through genetic and epigenetic regulation, to evade radiation and drug therapy after which they repopulate themselves, regenerating the tumor and leading to distant metastatic disease (Abou-Antoun et al Neurotherapeutics. 2017).
It is crucial to devise tailored, targeted therapeutic approaches to cancer treatment and to reduce the recurrence of malignant, metastatic disease by eradicating cancer stem-cells.
Notably, the cancer stem-cell driving protein, L1-CAM, that we reported to be over-expressed in the neuroblastoma tumorspheres, is being used in immunotherapy, specifically the groundbreaking “chimeric antigen receptor redirected T Lymphocytes (CAR-T)” approach. In this highly specific and targeted therapy, scientists educate the T cells to attack L1-CAM protein epitopes on all neuroblastoma cells (Hong, H. et al. J Immunotherapy. 2014), which would also eradicate the stem-like tumorspheres.
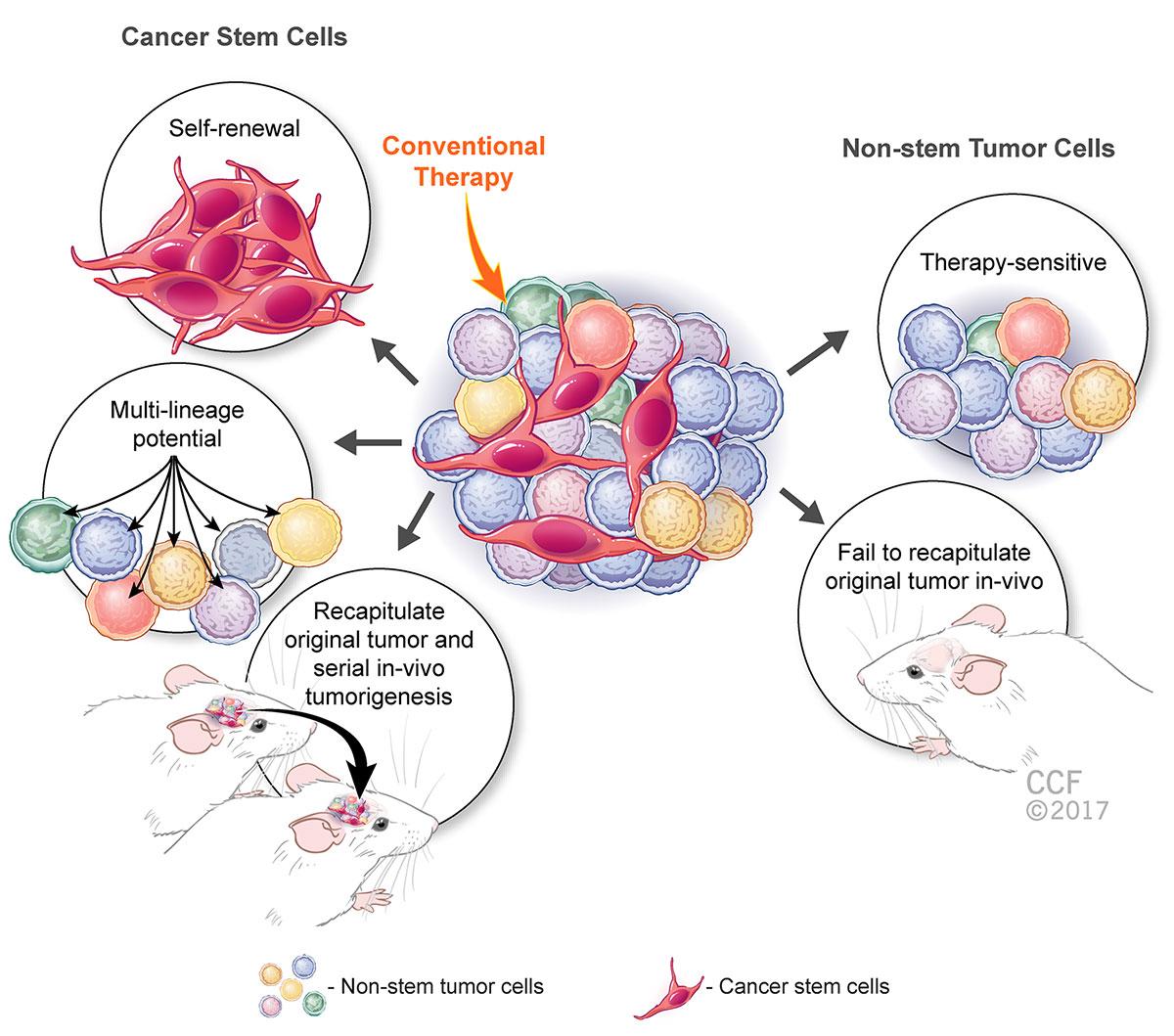
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2017. All Rights Reserved.
In the illustration above, while the non-stem cancer cells are sensitive to therapeutic manipulation and cannot regrow the same tumor if injected into the mouse brain, cancer stem-cells have the capacity to self-renew, give rise to multiple lineages and regrow the same tumor over and over again, also known as serial in vivo tumorigenesis.
I like to use the “mint-plant” analogy to describe this phenomenon. The more you trim the mint leaves, the stronger the roots become, going deeper into the soil and fortifying the ever-growing mint bush above the ground; the only way to eliminate any further growth of the mint bush is to pull out the roots altogether from the ground.
With aggressive cancers, the more we eradicate the bulky mass of the tumor, which primarily contains the non-stem, therapy-responsive cancer cells, the more we are empowering the stem-cell “roots” hidden deep within the core that continue to give rise to more aggressive and malignant, treatment-evasive cancers.
Previous work identified and described this “stem-like” transient cell phenotype with plasticity (changes in response to environmental factors) in a mouse tumor model of neuroblastoma (Chakrabarti L. et al. Front. Oncol. 2013). Our research group work that is underway shows that molecular analysis of these transitional cells revealed proteins that are exclusively expressed by the treatment-resistant, stem-like cancer tumorspheres. These proteins are reported to be highly associated with increased malignancy and metastasis in many tumors including central nervous system tumors, breast cancer, and hematologic tumors among others.
The illustration below highlights the factors that may be driving this phenotypic plasticity of the tumorspheres, including unfavorable culture condition such as low pH, serum deprivation and hypoxia. These conditions may even be caused by pharmaco-therapeutics and radiotherapy, which most cancers are subject to.
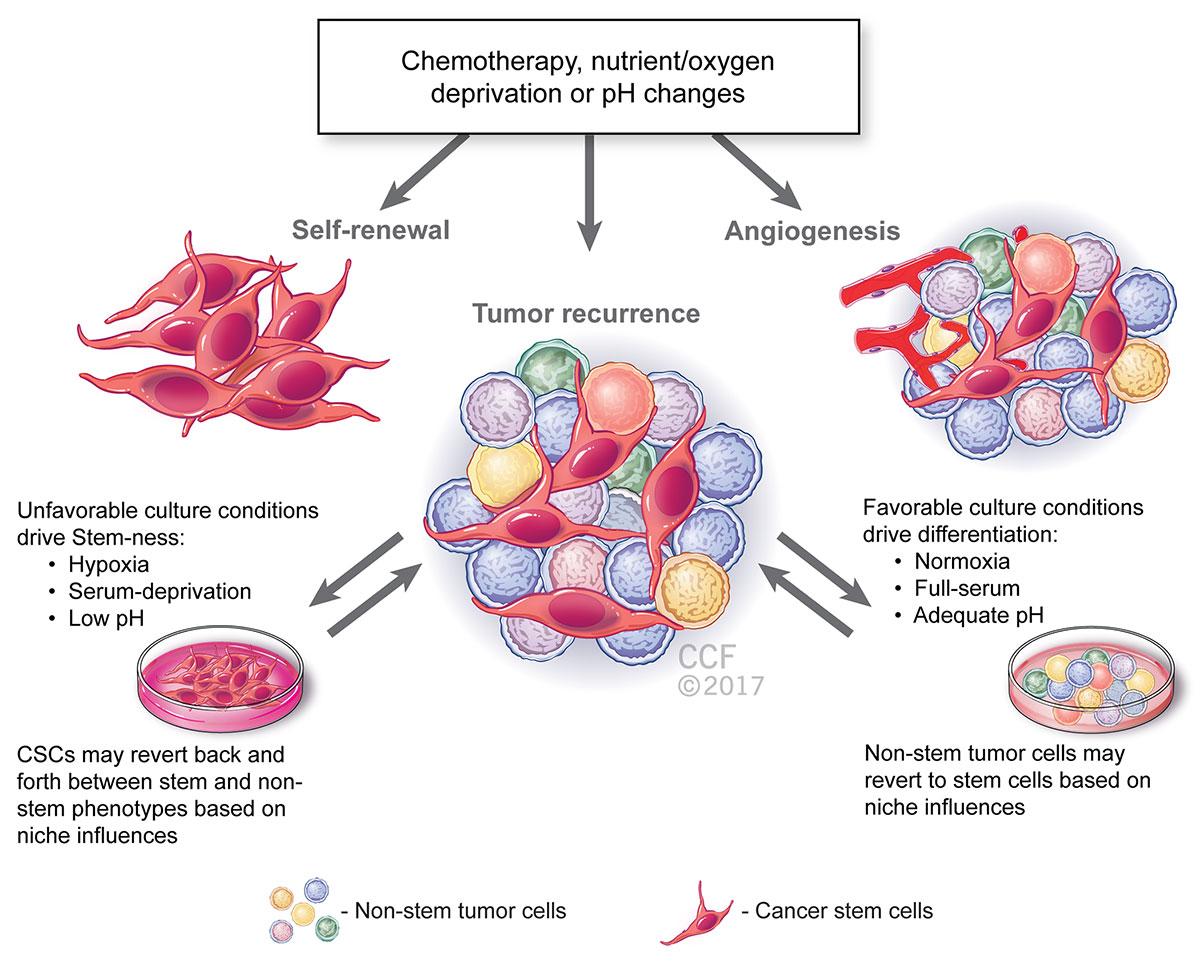
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2017. All Rights Reserved.
By conducting extensive molecular and proteomic analyses on these proteins of interest, one would expect to find targets for destroying tumor-initiating, treatment-resistant and highly malignant sub-populations of cancer stem-cells within the bulky tumor mass.
A sound understanding of the mechanisms that govern the malignant phenotype of these treatment-evading tumorspheres is very important to hopefully bring cancer researchers one step closer to a complete cure, with long-term, cancer-free survival in children.
My long-term career objective is to make a significant impact on the outcome of cancer treatment and quality of life in these children. I hope to advance my scholarly achievements by securing intra- and extra-mural funding for my research and continuing to educate and train young scientists to take on the challenge of cancer research towards finding effective cures. Along the same lines, the anticipated graduate program in pharmaceutical sciences at the School of Pharmacy would be invaluable to the scholarly achievements that my colleagues and I would accomplish in this domain at LAU.